Taken from http://www.sizes.com/units/atomic_mass_unit.htm

History of the atomic mass unit
Stanislao Cannizzaro (1826–1910), the pioneer in this field, adopted the hydrogen atom as a standard of mass and set its atomic weight at 2. Others accepted the idea of using a specific atom as a standard of mass, but preferred a more massive standard in order to reduce experimental error.
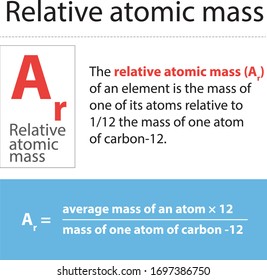
The IUPAC definition of relative atomic mass is: An atomic weight (relative atomic mass) of an element from a specified source is the ratio of the average mass per atom of the element to 1/12 of the mass of an atom of 12 C. Hence the relative atomic mass of the mass m is defined as: (3) A r = m m u The quantity is now dimensionless. As this unit is confusing and against the standards of modern metrology, the use of relative mass is discouraged.
As early as 1850, chemists used a unit of atomic weight based on saying the atomic weight of oxygen was 16. Oxygen was chosen because it forms chemical compounds with many other elements, simplifying determination of their atomic weights. Sixteen was chosen because it was the lowest whole number that could be assigned to oxygen and still have an atomic weight for hydrogen that was not less than 1.
The 0=16 scale was formalized when a committee appointed by the Deutsche Chemische Gesellschaft called for the formation of an international commission on atomic weights in March 1899. A commission of 57 members was formed. Since the commission carried on its business by correspondence, the size proved unwieldy, and the Gesellschaft suggested a smaller committee be elected. A 3-member International Committee of Atomic Weights was duly elected, and in 1903 issued its first report, using the 0=16 scale.5
Relative Atomic Mass
Taking isotopes into account

The discovery of isotopes complicated the picture. In nature, pure oxygen is composed of a mixture of isotopes: some oxygen atoms are more massive than others.
This was no problem for the chemists’ calculations as long as the relative abundance of the isotopes in their reagents remained constant, though it confirmed that oxygen’s atomic weight was the only one that in principle would be a whole number (hydrogen’s, for example, was 1.000 8).
Physicists, however, dealing with atoms and not reagents, required a unit that distinguished between isotopes. At least as early as 19276 physicists were using an atomic mass unit defined as equal to one-sixteenth of the mass of the oxygen-16 atom (the isotope of oxygen containing a total of 16 protons and neutrons).
In 1919, isotopes of oxygen with mass 17 and 18 were discovered.7 Thus the two amu’s clearly diverged: one based on one-sixteenth of the average mass of the oxygen atoms in the chemist’s laboratory, and the other based on one-sixteenth of the mass of an atom of a particular isotope of oxygen.
In 1956, Alfred Nier (at the bar in the Hotel Krasnapolski in Amsterdam) and independently A. Ölander8, both members of the Commission on Atomic Masses of the IUPAP, suggested to Josef Mattauch that the atomic weight scale be based on carbon-12. That would be okay with physicists, since carbon-12 was already used as a standard in mass spectroscopy. The chemists resisted making the amu one-sixteenth the mass of an oxygen-16 atom; it would change their atomic weights by about 275 parts per million. Making the amu one-twelfth the mass of a carbon-12 nucleus, however, would lead to only a 42 parts per million change, which seemed within reason.
Mattauch set to work enthusiastically proselytizing the physicists, while E. Wichers lobbied the chemists.9 In the years 1959–1961 the chemists and physicists resolved to use the isotope carbon-12 as the standard, setting its atomic mass at 12.
By international agreement the mass of an atom of carbon-12 is given as 12 unified atomic mass units (u). Therefore, 1 u is 1/12th the mass of an atom of carbon-12.
In most chemistry work, you can:
• neglect the tiny contribution to atomic mass from electrons
Relative Atomic Mass Of Oxygen
• take both the mass of a proton and a neutron as being equal to 1 u.
In a pure isotope all the atoms have the same atomic structure and the same mass.
Relative isotopic mass is then simply the mass number of the isotope, e.g. the relative isotopic mass of 32Sis 32.
However, most elements contain a mixture of isotopes, each with a different mass number. To find the relative atomic mass of an element we must know:
The relative isotopic mass of each isotope.
The proportion of each isotope in the element.
For example:
Copper has 2 isotopes, 63Cu and 65Cu. The proportions of each isotope are 69.17% and 30.83%.
These can be rounded to 70% and 30%.
The relative atomic mass of Copper is therefore (70/100 x 63) + (30/100 x 65) = 63.6
There is no unit as it is a relative value.
Molecular Mass (Mr) is the sum of all the relative atomic masses for all the atoms in a given formula.

For covalent compounds it is called the Relative Molecular Mass.
For ionic compounds it is called the Relative Formula Mass.
Methane (CH4)for example, contains 1 Carbon atom and 4 Hydrogen atoms.
Therefore, the molecular mass is calculated as follows:
C = 12, H = 1
Relative Atomic Mass Of Sodium
CH4 = C + (H x 4) = 12 + (1 x 4) = 16




