Atomic Mass of Nickel Atomic mass of Nickel is 58.6934 u. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The unit of measure for mass is the atomic mass unit (amu). Name: Nickel: Symbol: Ni: Atomic Number: 28: Atomic Mass: 58.6934 atomic mass units: Number of Protons: 28: Number of Neutrons: 31: Number of Electrons: 28: Melting Point.
The Iron Triad is composed of three elements: iron (Fe), cobalt (Co), and nickel (Ni), which share similar chemical and physical characteristics. They are found adjacent to each other in period 4 of the periodic table.
Introduction
The Iron Triad is known for possessing ferromagnetic elements similar to gadolinium (Gd), and neodymium (Nd). These types of ferromagnetic elements have the ability to create a large magnetic pole due to their unpaired electrons. When one of these elements is inside an environment where the temperature is at its individual Curie Point (Tc), however, the specific paramagnetic lining of atoms is broken down by the energy within the element and ferromagnetism is lost. The (Tc)'s for iron, cobalt, and nickel are 768°C, 1121°C, and 354°C respectively and are taken advantage of to make use of these elements in industry. In addition, elements in the iron triad are commonly combined with carbon and each other to create various types of alloys. It is because of these magnetic properties and use in alloys that the three elements are typically grouped together and labeled as the 'Iron Triad'. While very similar in magnetic properties and reaction, these elements are also very unique and used differently in both nature and industry.
Iron
Iron (Fe) is a transition metal with an atomic weight of 55.845 and an atomic number of 26. Its most common and comfortable oxidation state is +3 and is usually a shiny silver color. While it can be found in the sun and the stars, it is extremely common on planet earth and its abundance in the earth’s crust is 4.7%. The pure metal form of iron is rarely found due to its ability to easily react with other metals and environments.
Iron's most common use in worldwide industry is to be used in alloys and steels for everyday use, some which even contain other elements in the Triad. By combining metals like iron with carbon to make alloys, very strong materials can be made that are used for steel housings, supports in television tubes, and other important products. Pure iron is available commercially in the United States and production of about 500 million tons of Iron is created every year throughout the world.

From a biological perspective, iron is essential to organisms since it’s needed for the creation of hemoglobin. Humans need hemoglobin since these red pigments supply oxygen to our body through red blood cells. To increase intake of iron, foods like eggs, groundnuts and green leafy vegetables should be consumed. Without iron, our bodies would not be able to form hemoglobin and an organism would fail to survive.
Cobalt
Cobalt (Co) is a transition metal with an atomic weight of 58.93 and an atomic number of 27, right in between iron and nickel. Cobalt, however, is not as abundant as iron and only makes up of about 0.0020% of the Earth’s crust. This makes cobalt a little more rare and valuable than the other members of the triad because it is still used heavily in international industry. It is for this reason that this hard gray element is mined for and traded around the world.
Like iron, this element is commonly combined with other metals to create alloys. Superalloys that contain cobalt are used for parts of gas turbine engines that benefit both commercial and military devices. In current times, however, the rise of rechargeable batteries has made demand for the element rise to new heights since it is commonly used for electrodes in this relatively new technology. While these are its most popular applications, cobalt is also utilized in the production of petroleum, dyes, magnets, and electronics due to its ferromagnetic properties.
Cobalt and its alloys have recently made strides in the biomedical sphere by playing a large role in neurological, dental, orthopedic, and cardiovascular implant devices. This is due to the fact that the alloys function well with their corrosion resistance, mechanical properties, and biocompatibility.
Nickel
Nickel (Ni), a transition metal with an atomic weight of 58.69 and an atomic number of 28, is usually recognized by its silvery shine with a hint of gold color. It was first discovered by Axel Fredrik Cronstedt in 1751 and named nickel from the ore 'kupfernickel'. Although nickel is not extremely common, it still ranks as #24 on the list of the most abundant elements on earth.
This element is most abundant in Canada and about 300 million pounds are used in America every year. Nickel is most commonly used as a key ingredient in low alloy steels, stainless steels, and cast irons. In fact, four out of five of these 300 million pounds of Nickel go to this type of alloy making. Like cobalt, it shares the unique characteristic of corrosion resistance and is ideal for corrosion and heat resistant coatings, magnetic alloys, and controlled-expansion alloys.
Over 2000 years ago, the Bactrian civilization in Western Asia used a 75:25 alloy of copper and nickel for its coins. A modern US nickel has the same composition, but a modern Canadian nickel is nickel-plated steel and contains only 2.5% nickel by mass.

New nickel-cadmium rechargeable batteries have also brought the use of nickel into a new light. Like cobalt, the turning of the millennium and the increased use of rechargeable batteries has given nickel a new major use that will likely surpass others in the future. Nickel-cadmium batteries have improved the performance of cordless power tools, portable computers, and other portable electronic devices.
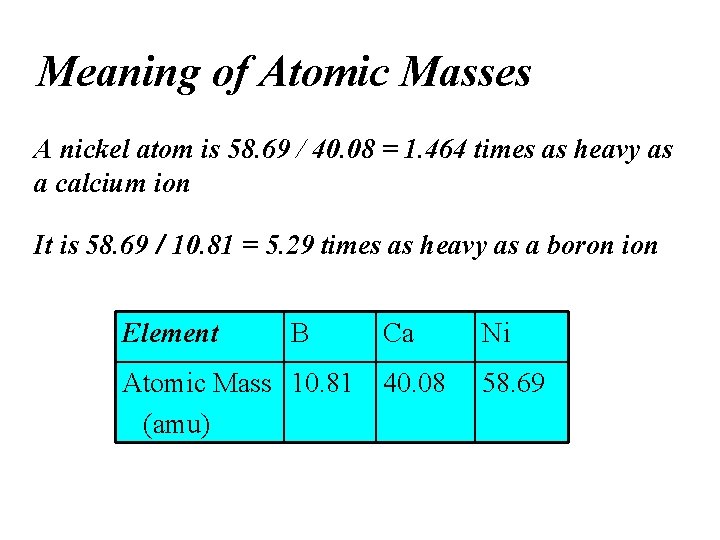
Iron | Cobalt | Nickel | |
Atomic Number | 26 | 27 | 28 |
Mass | 55.85 | 58.93 | 58.69 |
Electron Configuration | 3d64s2 | 3d74s2 | 3d84s1 |
Metallic radius, pm | 124 | 125 | 125 |
Ionization Energy, kJ mol-1 | 762.5 | 760.4 | 737.1 |
| 759 | 758 | 737 |
| 1561 | 1646 | 1753 |
| 2957 | 3232 | 3393 |
E°, Vb | -0.440 | -0.277 | -0.257 |
Common (+) Oxidation States | 2, 3, 6 | 2, 3 | 2, 3 |
Melting Point (°C) | 1530 | 1495 | 1455 |
Boiling Point (°C) | 2862 | 2927 | 2732 |
Density, g cm-3 | 7.87 | 8.90 | 8.91 |
Electrical Conductivitye | 16 | 25 | 23 |
References
Atomic Mass Of Nickel Ii Chloride
- Cracolice,Mark S. and Peters, Edward I. - Introductory Chemistry: An Active Learning Approach
- John A. Disegi, Richard L. Kennedy, Robert Pilliar - Cobalt-Base Alloys for Biomedical Applications
- Joseph R. Davis, ASM International - ASM Specialty Handbook: Nickel, Cobalt, and Their Alloys
- Kim B. Shedd - The United States Geological Survey 2006 Minerals Yearbook
- Petrucci, Harwood, Herring, and Madura - General Chemistry 9th Edition
- Walter H. Kohl - Handbook of Materials and Techniques for Vacuum Device
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
Atomic Number Of Nickel
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.

The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.




